
CAR T-cell therapy is a type of immunotherapy that uses the patient’s own T cells from the immune system for treatment. CAR stands for the “chimeric antigen receptor” these cells carry to recognise cancer cells. The entire CAR T-cell treatment process involves several steps, but usually only takes place once. CAR T-cell therapy is another treatment option, particularly for patients with certain blood cancers. These patients may previously have had a poor prognosis and did not respond to standard care treatments.1,2
CAR T-cells are produced individually for each patient using T cells taken from the patient’s own body.1 T cells are part of the body’s immune system to help fight off infections like viruses and bacteria, while also targeting the body’s abnormal cells, including cancer cells. However, cancer cells often develop mechanisms to evade detection by the immune system, allowing them to escape these immune responses.3
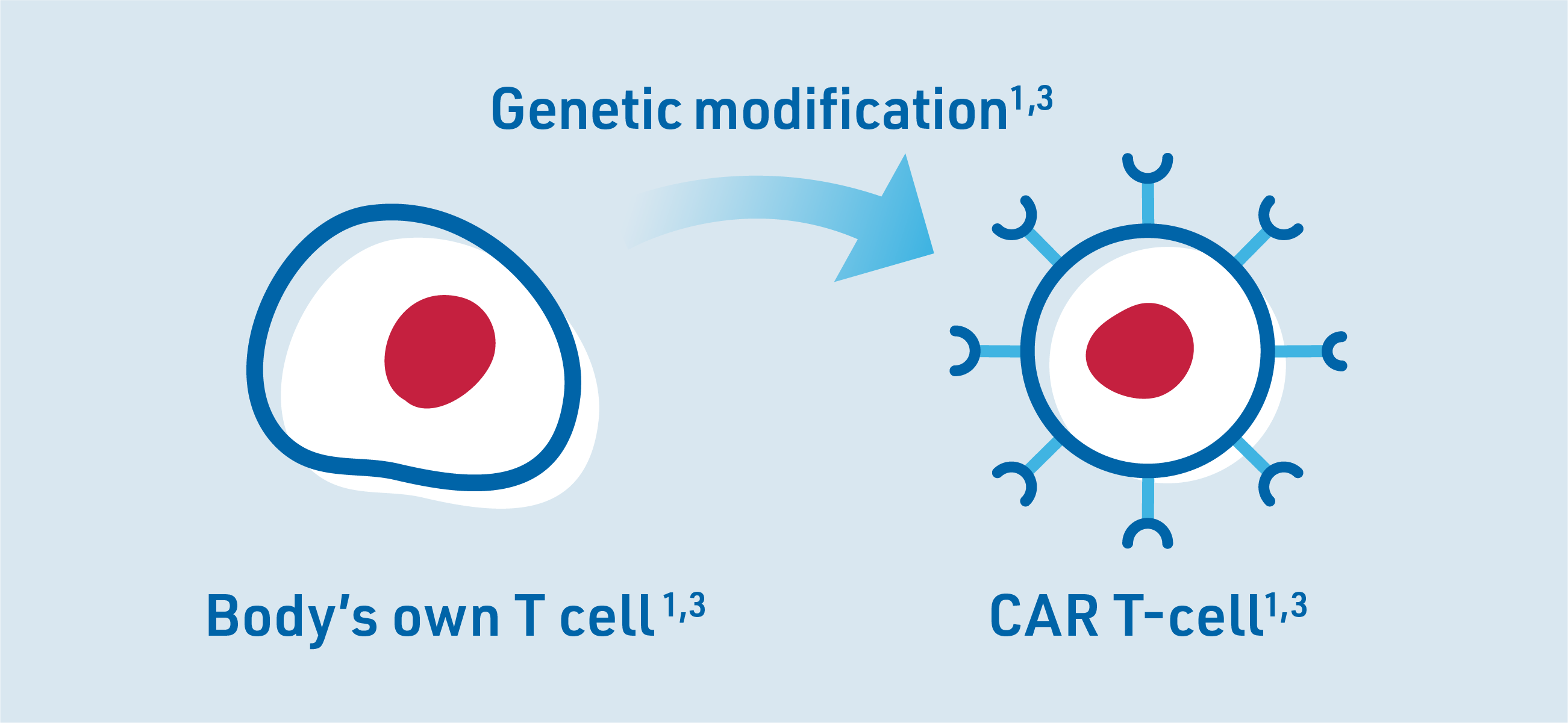
Through genetic engineering, the T cells are modified to have a chimeric antigen receptor (CAR) on their surface so they can identify cancer cells as a threat to the body. The modified T cells are called CAR T-cells.3
Whether CAR T-cell therapy is a suitable option for you depends largely on the type of cancer and the prior treatments you have received. It is important to speak with your haematologist or oncologist to find out if CAR T-cell therapy is an option for you.
CAR T-cells were developed to help the immune system recognise and destroy cancer cells.
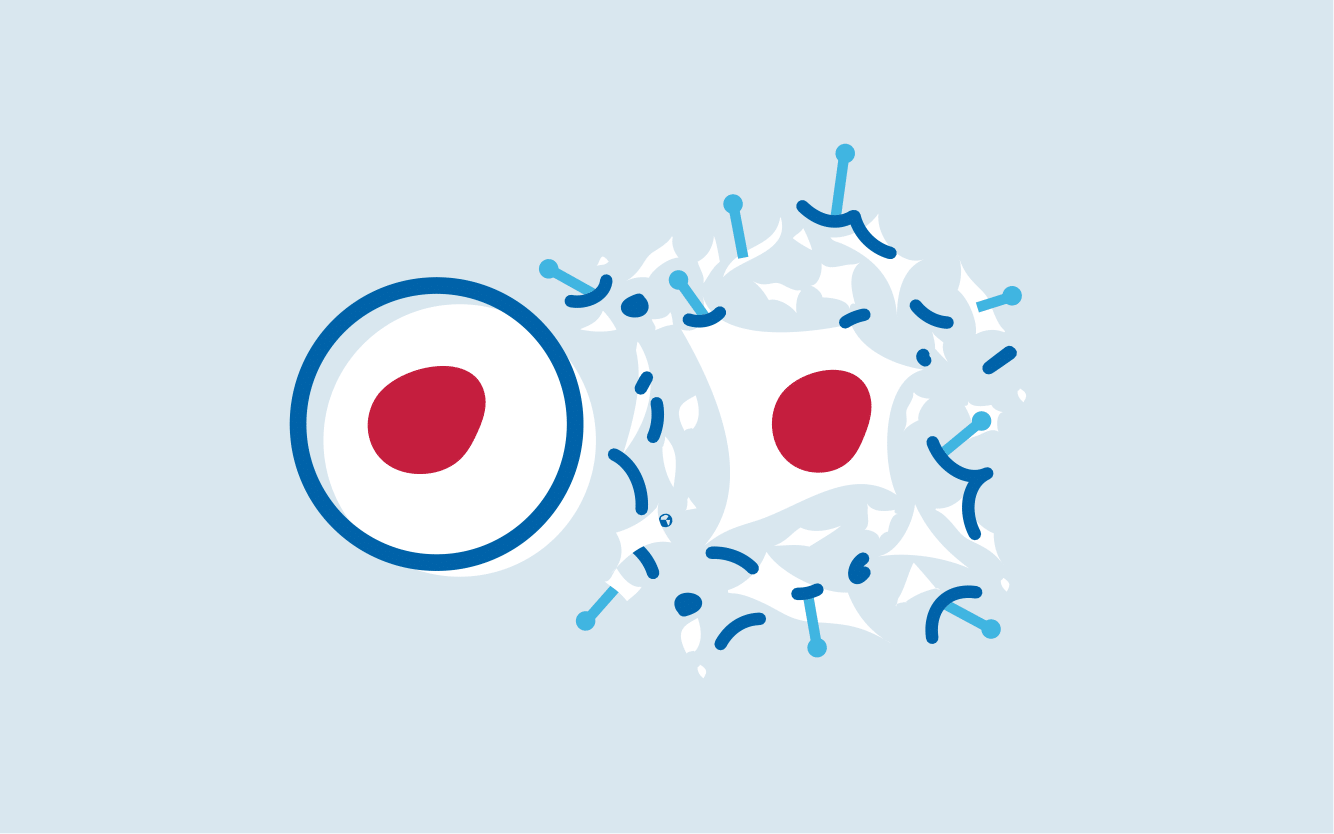
T cells are a vital component of the body’s immune system, responsible for defending against pathogens and attacking mutated cells, including cancer cells.
However, some cancer cells can hide from T cells and multiply unnoticed in the body. This is where CAR T-cell therapy comes in.
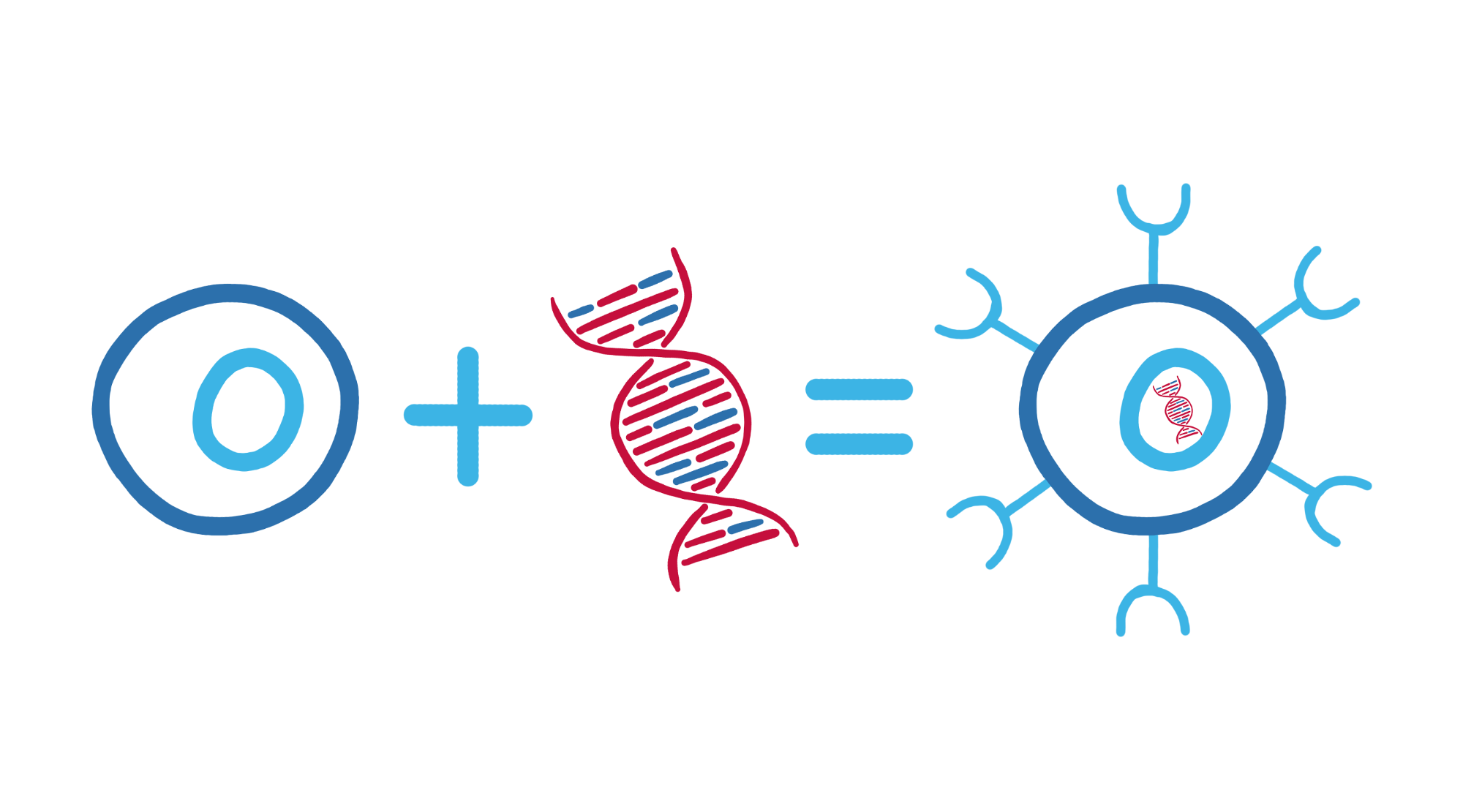
In CAR T-cell therapy, the patient’s own T cells are collected, genetically modified and then infused back into the patient’s body (via a drip into a vein).
These modified T cells are called CAR T-cells because they now have the chimeric antigen receptor (CAR) on their surface.
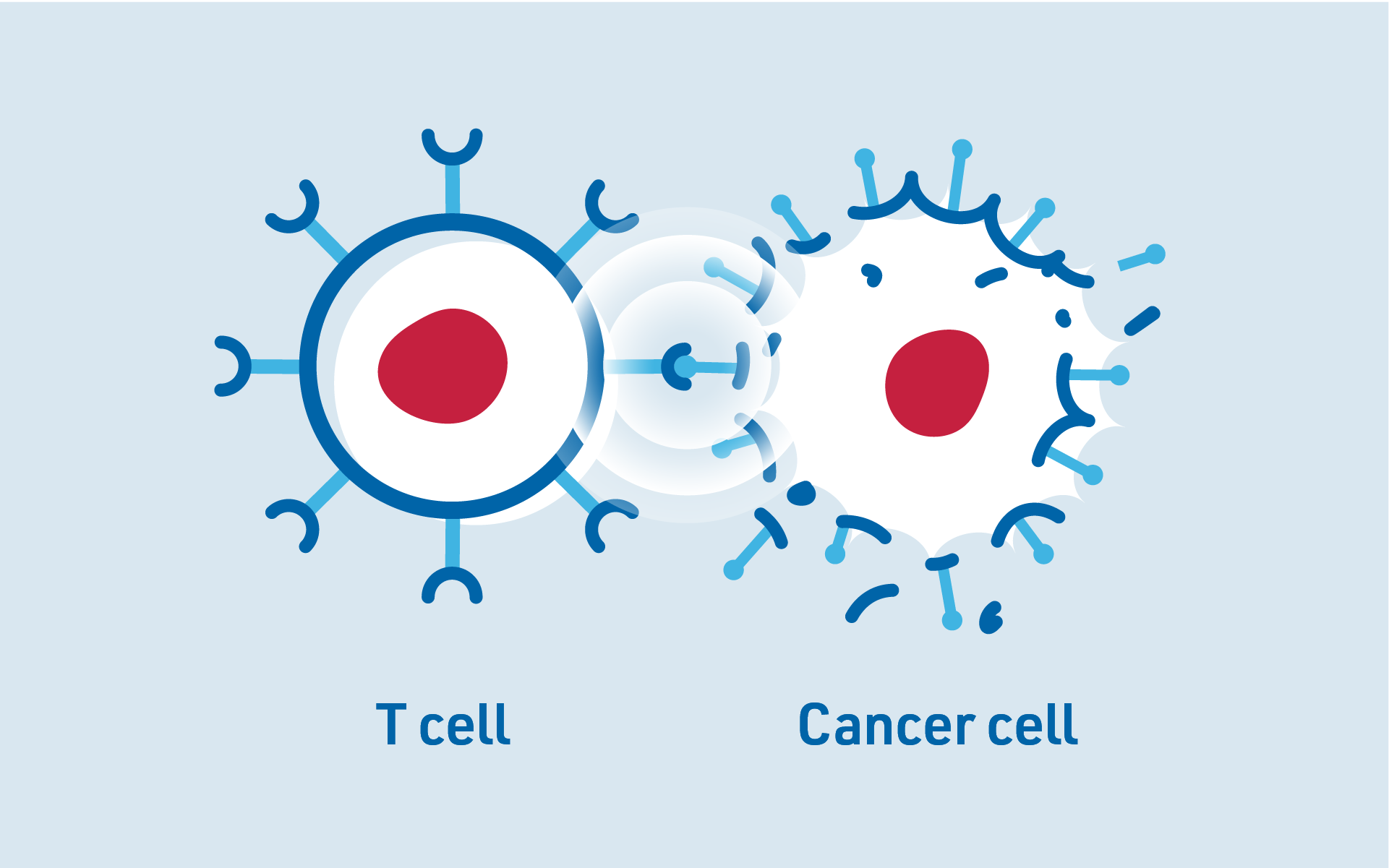
The CAR helps CAR-T cells detect and destroy cancer cells by recognising specific markers on the surface of these cells, making it easier for the immune system to attack the cancer cells.
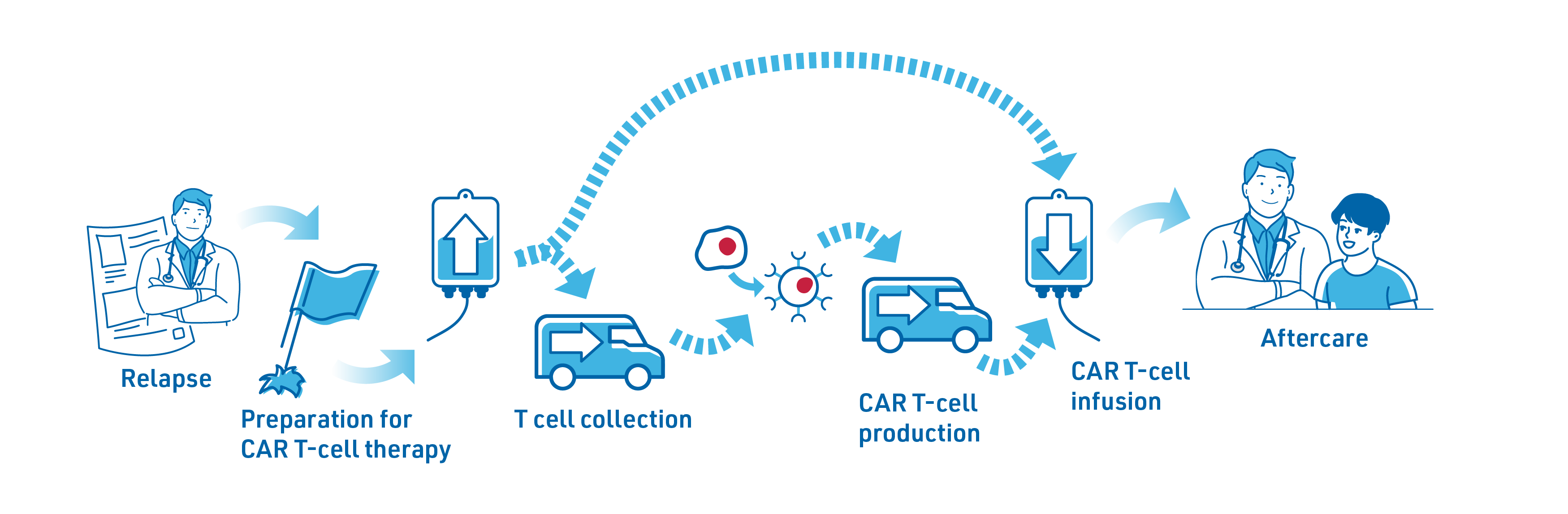

If your lymphoma relapses, the physician will discuss available treatment options with you. If CAR T-cell therapy is considered a possibility, they will discuss your case with experts from the specialist CAR T-cell therapy centre who will review the results of any tests or scans you have received to decide if the treatment is right for you.2
Prior to starting CAR T-cell therapy, the following preliminary tests will generally be performed:2
These are important to monitor the progression of the cancer and assess if you are fit enough to receive the treatment.2
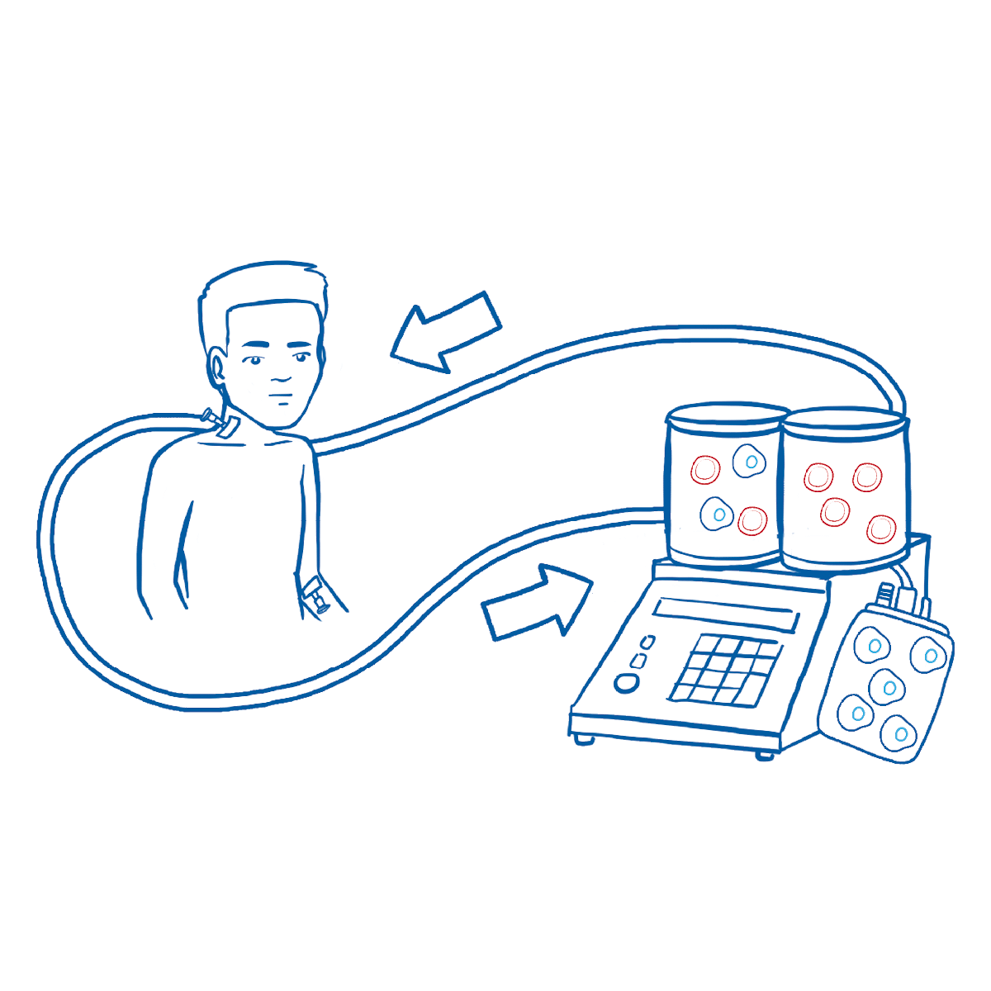
T cells are collected through a process called apheresis in which white blood cells, including T cells, are filtered out of the blood for collection. The remaining blood will be returned to the patient’s bloodstream. This procedure usually takes about takes about 4-6 hours.2,3,7
The collected blood cells are sent to a manufacturing facility to produce the CAR T-cells. There, the T cells are genetically modified with the CAR, multiplied and sent back to the treatment centre.2
Production and logistics of CAR T-cells can take a few weeks during which the patient’s disease could progress. Bridging therapy is an option to keep the lymphoma under control, but it may not always be required. If your doctor recommends bridging therapy, it may include chemotherapy or radiotherapy.2
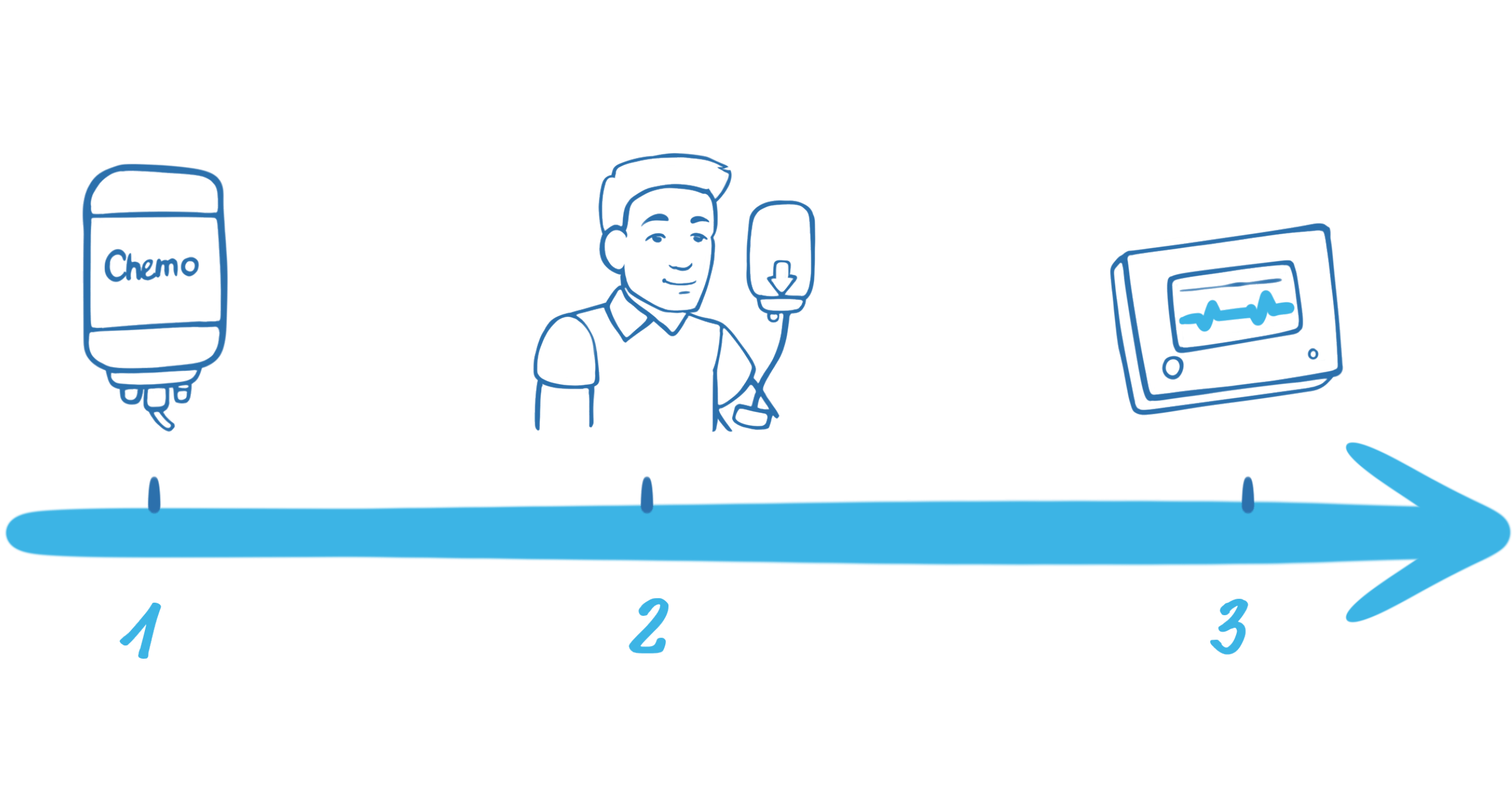
1. Lymphodepleting chemotherapy2,3
Lymphodepleting chemotherapy is given over 3 days to lower the number of white blood cells and prepare your body for the CAR T-cells so they can work effectively.2
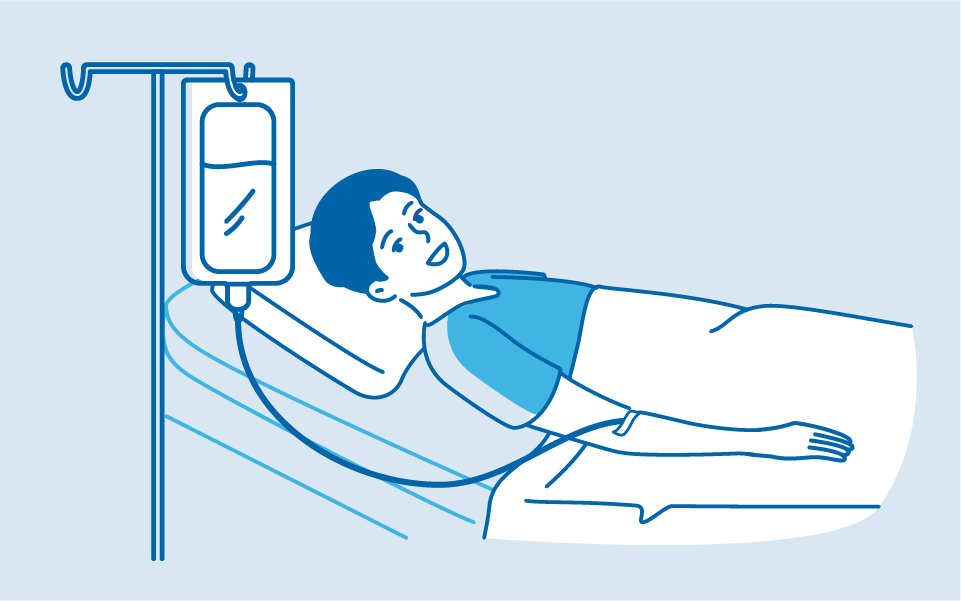
2. CAR T-cell Infusion
The CAR T-cell infusion is through a drip into a vein and takes up to 30 minutes.7
3. Monitoring in the hospital
After the infusion, the CAR T-cells become active in the body and start attacking the cancer cells. As CAR T-cells are living cells, they continue to multiply in your body so you have an ongoing supply targeting your cancer cells.3 During this period, you will be carefully monitored in hospital for 7–10 days (time may vary depending on physician discretion).3
It is important to know that CAR T-cell treatment may cause side effects that can be serious and occasionally life-threatening.2 These side effects can include:2
Because of the seriousness of these side effects, it is important to have follow-up observations to help ensure you receive treatment quickly if you develop any symptoms.2
After discharge from the inpatient hospital stay, patients are required to stay close to the hospital for a few weeks.2 Staying close to the CAR T-cell therapy centre is important to ensure any delayed side effects are attended quickly and effectively.2 Friends or relatives can be of great support staying with you during this time, helping with trips to the treatment centre or noticing potential side effects. After this close monitoring period, the treating haematologist/oncologist can then take over further follow-up.2
Disease assessments will be performed regularly, usually at 1 month and 3 months after receiving your CAR T-cell therapy.2
CAR T-cell therapies can effectively fight some serious types of cancer, but like other cancer therapies, they are sometimes associated with side effects. Among the most significant are cytokine release syndrome (CRS, sometimes called a cytokine storm) and neurological side effects. In most cases, these and other associated side effects can be managed with medication.1,3,8
To fight cancer cells, CAR T-cells release messenger substances, also known as cytokines, similar to normal T cells. If too many cytokines are released, this can lead to cytokine release syndrome.1,3 Symptoms can include fever, nausea, cardiovascular disorders, diarrhoea and vomiting, fatigue and shortness of breath.3,8 Although it can be treated, cytokine release syndrome can be serious or life threatening if not treated early,8,9 which is why your treatment team will pay particular attention to any symptoms.
CAR T-cell therapy may also cause neurological side effects, such as encephalopathy (brain dysfunction), headaches, confusion or delirium. Common symptoms can include speech difficulties, tremors, agitation and dizziness. These effects can be serious or even life threatening, so it is important to immediately report any symptoms to your treatment team.7,9
CAR-T cells can sometimes attack healthy immune cells along with cancer cells, weakening the immune system and making patients more susceptible to infections. This immune deficiency can be managed with immunoglobulins, which are proteins that help the body fight off harmful pathogens.7,9
Administration of CAR T-cell therapy requires care from treatment selection to follow-up care and monitoring.7
To minimise the safety risks associated with CAR T-cell therapy, treatment with CAR T-cells is only carried out in centres with a specialised trained healthcare team.7,9 The medical staff are appropriately trained, and specific medication is available to treat any side effects.
Side effects can also occur after the patient has already been discharged from the hospital, which is why it is necessary to stay near the treating hospital post the CAR T-cell infusion so that any side effects can be treated quickly.7

References: