
Follicular lymphoma (FL) is a disease of the lymphatic system and a type of non-Hodgkin lymphoma. It develops from B lymphocytes that proliferate uncontrollably, primarily in the lymph nodes and bone marrow.1,2
FL is a slow-growing (indolent) lymphoma. Sometimes, FL can transform into a fast-growing (aggressive) type of lymphoma.1,2

FL account for around 30% of all lymphomas.2 The incidence of FL varies by country; In Western countries, it is the 2nd most-common non-Hodgkin lymphoma, but in Asia, the incidence has historically been low at approximately 5–10%. Recently, however, the incidence has increased, and it is now thought to be the 2nd most-common low-grade B-cell lymphoma in Asian countries.3,4
The exact cause of FL is still unknown. While the tumour cells in FL show genetic changes, it’s not yet clear what triggers these changes.1
One common genetic alteration in FL is a chromosomal translocation known as t(14;18). This occurs when parts of chromosome 14 and chromosome 18 break off and switch places.2 This translocation causes cancerous B cells to multiply and grow uncontrollably.5
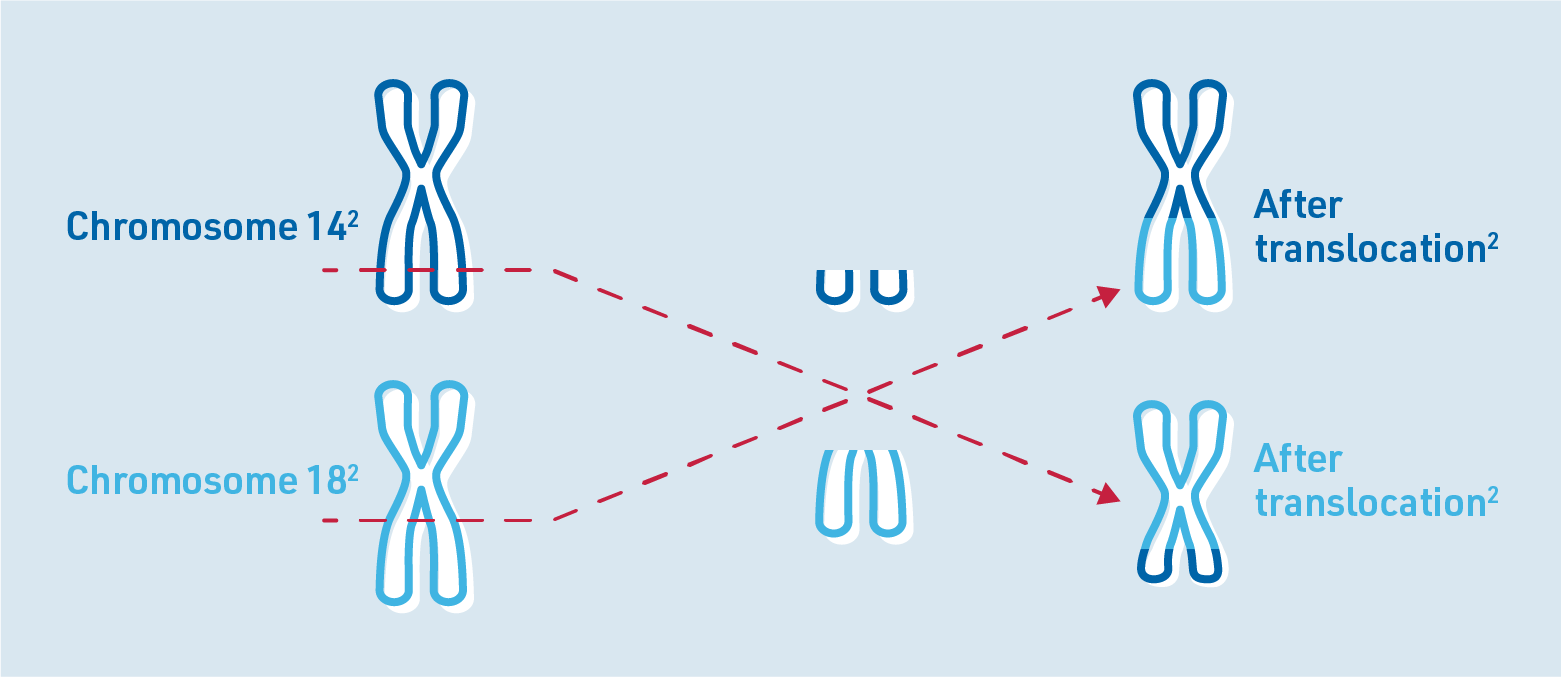

Chromosomes are structures in human cells that store genetic information. They consist of DNA and contain genes through which certain characteristics are inherited. Healthy human cells normally contain a total of 46 chromosomes. Each chromosome occurs twice. Human chromosomes are numbered from 1 to 23.6
Some studies have shown that there are several factors that increase the risk of developing FL. These include:7,8
Additionally, if the lymphoma affects the bone marrow, it can lead to:1
About one in five patients with FL develop B symptoms:1,2
If FL is suspected, a range of tests will be performed, including:1,2,9
WHO classifies FL into different grades based on the maturity and appearance of the lymphoma cells, specifically focusing on the number of large cells (called centroblasts) that are visible under a microscope. This process is referred to as grading.1,2 The grades for FL are:2
FL with grades 1, 2, or 3A is considered indolent (slow growing), while FL with grade 3B is classified as an aggressive (fast growing) lymphoma.1
How a patient with FL is treated depends on how far the disease has spread according to the Ann Arbor classification. There are four stages:2,5,10
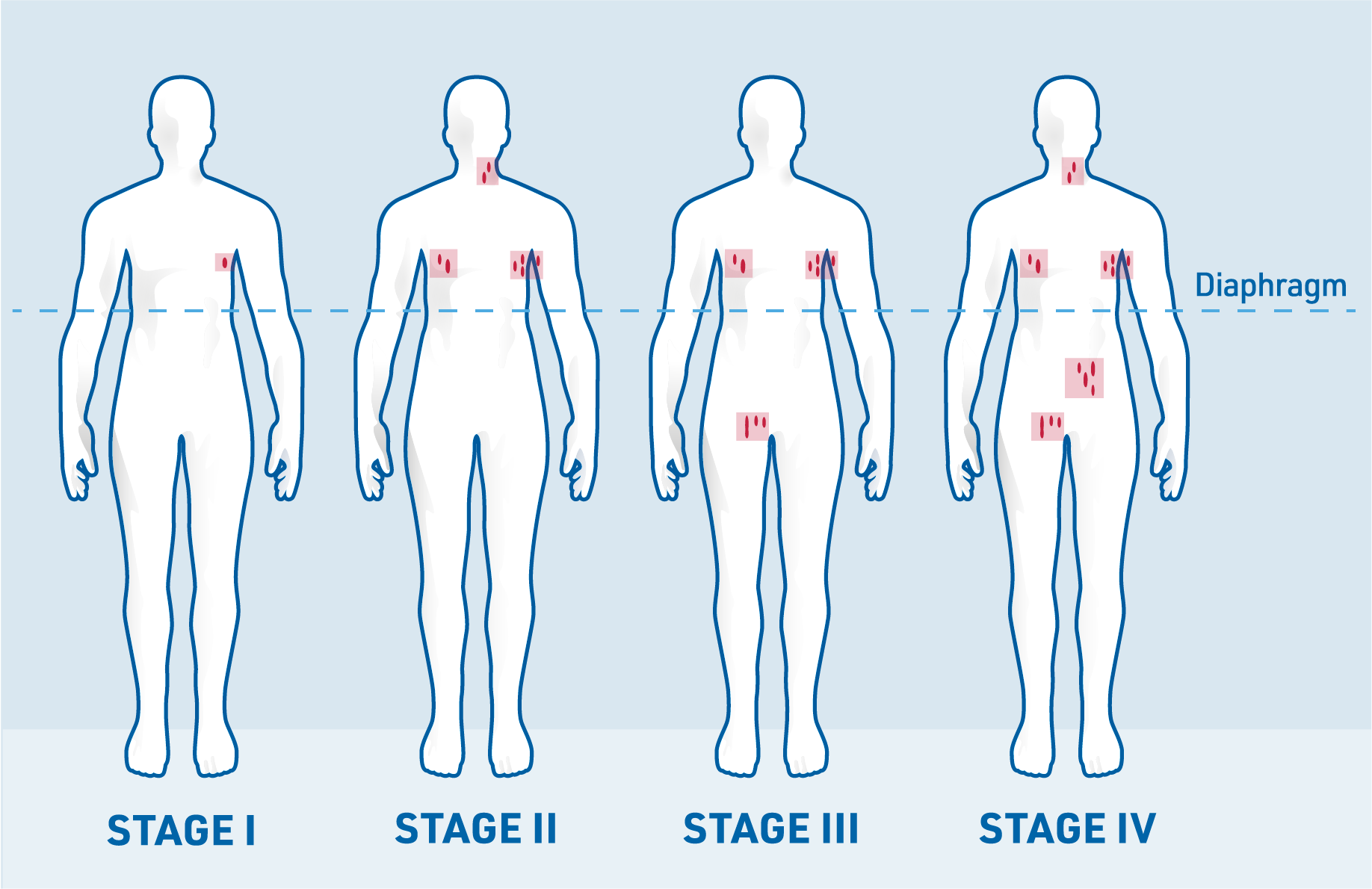
When assigning a stage, the presence or absence of B symptoms (fever, weight loss or night sweats) is also noted. If a patient does not have B symptoms, the stage is marked with an “A”. If B symptoms are present, the stage is marked with a “B”.9
If the lymphoma has spread to organs or tissues outside the lymph nodes, this is indicated with an “E” (for extranodal, meaning outside the lymph nodes).10
The Follicular Lymphoma International Prognostic Index (FLIPI) can help doctors estimate the likely course of the disease. To do this, the doctor considers the following risk factors:2,10
Each of these risk factors is given one point. The more of these risk factors that are present, the poorer the prognosis.2,10
Various treatment options are available for the treatment of FL, such as radiotherapy, chemoimmunotherapy, targeted therapies, stem cell transplant or CAR T-cell therapy.9 The choice of therapy depends on factors such as the stage of the disease according to the Ann Arbor classification and the patient’s general condition.1
For some patients, the doctor may recommend a “watch and wait” strategy in which the disease is monitored closely before starting treatment. This approach is often used for patients with slow-growing FL who aren’t experiencing significant symptoms.1
Treatment for FL depends on the extent of the disease when the diagnosis is made.1
In stages I and II according to the Ann Arbor classification, patients may be treated with radiation alone. If radiotherapy is not suitable, a short course of immunotherapy may be recommended.1,9
For patients diagnosed with advanced-stage FL (stages III and IV), the goal of treatment is to:1,2
The treatment approach depends on the patient’s symptoms and tumour burden:1,9
No symptoms, low tumour burden: In stages III and IV, if the patient has no symptoms and a low tumour burden, a watch and wait approach may be used. This approach is suitable because FL often progresses slowly (indolently). Research has shown that watch and wait does not negatively affect survival for patients without symptoms.1,11

Symptoms and/or high tumour burden: If the patient has symptoms (such as anaemia, fever or weight loss) and/or a high tumour burden, treatment should be started. The recommended therapy is chemoimmunotherapy, which combines chemotherapy with an immunotherapy such as rituximab or obinutuzumab. After this, maintenance therapy with the immunotherapy may be recommended for 2 years to prolong remission11,12
Tumour burden is a measure of the amount of cancer cells in the body. In a patient with FL, a high tumour burden may be indicated by several factors, including the presence of enlarged lymph nodes that measure over 7 cm in diameter.11
Stage III and IV FL is generally not curable, and it often recurs at some point. If a relapse occurs, another biopsy will be performed to check if the FL has transformed into a more-aggressive type of lymphoma.1
The choice of treatment for relapsed or refractory FL depends on a variety of factors including the type of prior therapy and maintenance therapy as well as how quickly the FL relapsed.1
If you have not had chemoimmunotherapy before, or you have had a long remission, it is often possible to receive chemoimmunotherapy again. If you did not respond or you relapsed quickly, the antibody may be changed.1,12
If a relapse occurs less than 2–3 years after starting chemoimmunotherapy, high-dose chemotherapy followed by an autologous stem cell transplant can be considered.13 In this type of transplant, the patient’s own stem cells are harvested, treated and returned to their body.14
For younger, fitter FL patients who have relapsed after an autologous stem cell transplant and/or who do not respond to chemotherapy, an allogeneic stem cell transplant may be considered.13 In allogeneic stem cell transplants, the stem cells come from a donor.14
CAR T-cell therapy is an innovative treatment that uses the patient’s own genetically modified T cells to target and kill cancer cells. The T cells are altered to express a chimeric antigen receptor (CAR) on their surface, enabling them to recognise cancer cells as a threat and attack them.9,11
Researchers have developed various new drugs for the treatment of lymphoma. Some of these new treatments have shown promising results in studies of patients with FL.1 The haematologist/oncologist will discuss with the patient the suitability of these new treatments.
After completing treatment for FL, patients typically enter a structured follow-up program. During the first 2 years, check-ups will usually occur every 3 to 6 months to monitor the patient’s health and detect any signs of recurrence.1,13
As FL patients often remain in remission for only a limited period of time, regular follow-up visits are crucial to detect any signs of relapse.1 If the disease returns, early detection allows for treatment to be initiated quickly. Follow-up care also helps doctors monitor for any late effects of treatment, such as heart problems or the development of secondary tumours.1
Follow-up examinations typically involve:
Medical history and physical examination: Your doctor will ask detailed questions about the patient’s current health and any symptoms. A thorough physical examination will also be performed13

Blood tests: Blood samples are taken to assess the levels of different blood cells, and other important blood markers are analysed13
Additional tests: Depending on the situation, further tests like CT or PET scans may be recommended to monitor for signs of relapse or other health concerns13
References: